![]()
Cobalt Oxide
Cobalt Oxide
Cobalt oxide is Black or gray crystal. It can absorb water and form hydrate, but has no fixed composition. It is easily reduced to cobalt by carbon, carbon monoxide or hydrogen. When the oxygen is lost above 900℃, it becomes cobalt monoxide, which can absorb oxygen at lower temperatures, and the crystal structure is unchanged. Soluble in acids and bases, almost insoluble in water. Density: 6.11 Melting point :895 °C.
Cobaltous Carbonate Application:
Mainly used for battery materials, magnetic materials, thermistors, catalyst mechanism for enamel.
It is used for the preparation of high purity analytical reagent, cobalt oxide and cobalt salt.
Used for lithium electron cathode material, cobalt oxide and cobalt salt preparation.
Cobalt Oxide
CAS No.: 1308-06-1
MDL No.:MFCD00010939
EINECS No.:215-157-2
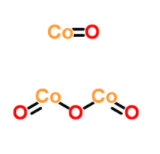
Molecular Formula :Co3O4
Molecular Structure :
Cobaltous Carbonate Specification :
| CHEMICAL INDEX | HR-I | HR-2 | HR-3 | HR-4 | HR-5 |
| Co % ≥ | 73.5±0.5 | 73.5±0.5 | 73.5±0.5 | 73.0 | 74.0 |
| Ni % ≤ | 0.05 | 0.05 | 0.05 | 0.15 | 0.05 |
| Fe % ≤ | 0.01 | 0.01 | 0.01 | 0.20 | 0.01 |
| Cu % ≤ | 0.003 | 0.003 | 0.003 | 0.10 | 0.05 |
| Zn % ≤ | 0.005 | 0.005 | 0.005 | 0.10 | 0.05 |
| Mn % ≤ | 0.005 | 0.005 | 0.005 | 0.10 | 0.05 |
| Na % ≤ | 0.01 | 0.01 | 0.01 | / | / |
| Mg % ≤ | 0.005 | 0.005 | 0.005 | / | / |
| Ca % ≤ | 0.005 | 0.005 | 0.005 | / | / |
| S % ≤ | 0.015 | 0.0015 | 0.0015 | / | / |
| Pb % ≤ | 0.005 | 0.005 | 0.005 | / | / |
| H2O % ≤ | 0.01 | 0.01 | 0.01 | / | / |
| Physical Index | |||||
| Apparent density g/cc | 0.50-1.2 | 0.50-1.2 | 0.50-1.2 | 0.50-1.2 | 0.50-1.2 |
| D50 μm | 3-6 | 6-9 | 9-15 | – | – |
Cobaltous Carbonate packaging:
25/50 kgs fibre board drum or iron drum with plastic bags inside
Cobaltous Carbonate Storage:
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat. Keep out of direct sunlight. The package is sealed. It should be stored separately from acids and edible chemicals, and should not be mixed. Storage areas should be equipped with suitable materials to contain leaks.








