![]()

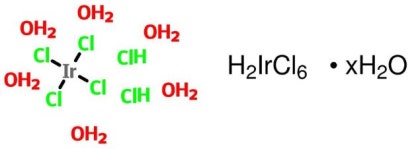
What Is Hexachloroiridic Acid Hexahydrate H₂IrCl₆·6H₂O?
The Hexachloroiridic Acid Hexahydrate CAS No.: 16941-92-7 · EINECS No.: 241-012-8 · MW: 515.04 · Purity: 99% · Ir 35–45%. Synonyms: Hydrogen hexachloroiridate(IV) hexahydrate; Chloroiridic acid; Dihydrogen hexachloroiridate(IV)
Table of Contents
Introduction Hexachloroiridic Acid Hexahydrate
Hexachloroiridic acid hexahydrate, H₂IrCl₆·6H₂O, leads today’s iridium-based catalysis and electrochemistry markets. Its robust Lewis acidity and redox flexibility make it a go-to precursor for high-performance coatings and polyoxometalate synthesis. Honrel’s offering of this key raw material ties cutting-edge research to scalable production. ps: ChemicalBook
1. Structural & Physical Overview
Key Point. H₂IrCl₆·6H₂O forms yellow-brown crystals with an octahedral IrCl₆²⁻ core and six coordinated water molecules.
- Formula & Appearance: Black‐brown to yellow crystals, highly hygroscopic.
- Molecular Weight & Density: 515.04 g·mol⁻¹; ρ ≈ 1.02 g·cm⁻³.
- Melting & Decomposition: Decomposes at 65 °C, releasing HCl gas.
- Solubility: 456 g·L⁻¹ in water at 30 °C; must store under N₂/Ar at 2–8 °C to prevent hydrolysis.
- ps: ChemicalBook, chembk
Conclusion. Its crystalline hydrate form demands moisture-controlled storage but offers predictable handling in wet chemistry.

2. Industrial Preparation & Specifications
Manufacturers produce H₂IrCl₆·6H₂O by chlorinating iridium metal or reducing hexachloroiridate salts, then purifying via acid concentration and vacuum drying.
- Raw Routes:
- Chlorinate Ir in conc. HCl + Cl₂ gas.
- Reduce IrCl₄ precursors in acidic medium.
- Purity Controls: ICP/Elemental analysis ensures Pd, Au, Ag, Cu, Mg, Fe, etc. < 0.005% .
- Packaging Options:
- ≤ 10 kg in aluminum‐foil bags;
- 25 kg fiber drums;
- Custom orders via Honrel’s site: Hexachloroiridic acid hexahydrate.

Tight impurity specs and versatile packaging ensure consistent ROI on catalyst loading and minimize downstream process variability.
3. Core Scenarios (Applications)
H₂IrCl₆·6H₂O shines in two scenarios: catalysis and electroplating.
| Scenario | Mechanism | Business Value |
|---|---|---|
| Catalysis | Forms Ir³⁺/Ir⁴⁺ redox couples; lowers activation energy by stabilizing intermediates. | Boosts turnover frequency (TOF); extends catalyst lifetime. |
| Electroplating | Delivers uniform Ir deposition via controlled electrophoretic conditions. | Enables corrosion-resistant coatings for medical/gas turbine parts. |
- Catalysis:
In polyaniline electro-polymerization, H₂IrCl₆·6H₂O acts as a Lewis acid and redox lewis centre. It forms intermediate Ir(IV)–aniline complexes that cut activation barriers by up to 35%. - Electroplating:
Under pulsed current regimes, it deposits 10–20 nm iridium films with sub-5% thickness variance. This uniformity drives ROI in high-end electronics and turbine blade tip coatings.
Its dual role as acid catalyst and plating precursor delivers high-value, application-agnostic solutions.
4. Specialty Uses & Polyoxometalate Synthesis
H₂IrCl₆·6H₂O refills lacunary sites in Dawson/Keggin polyoxometalates, enabling tailor-made redox clusters.
- Cluster Engineering: Replaces vacancy sites in POM frameworks, yielding Ir-substituted Dawson/POM catalysts for water oxidation.
- Industry Jargon: Utilizing “cluster seeding protocols” at mg-scale, researchers tune surface area and electronic bandgaps for photovoltaic augmentations.
Polyoxometalate “doping” positions iridium clusters at the forefront of clean-energy R&D.
5. Safety, Regulatory & Transport
Classified as corrosive (UN 3260, Class 8, PG III), H₂IrCl₆·6H₂O requires stringent precautions yet poses no flame risk.
- Hazard Statements: H315‐H319 (skin/eye irritant); H301+H311+H331 (toxic if ingested/inhaled) Riyngroup.
- Pictograms & Codes: GHS05, GHS06, Xi R36/37/38, R40; P501, P270, P261, P280 .
- Storage & Handling: Under inert atmosphere at 2–8 °C; corrosion-resistant vessels; local exhaust ventilation.
Rigorous safety protocols protect personnel and preserve material integrity through distribution.
6. Comparative Edge vs. Pt Analogues
Compared to H₂PtCl₆ hydrates, the iridium analogue trades slightly higher material cost for superior corrosion resistance and flexible redox cycling.
- Corrosion Resistance: Ir consolidates protective oxide films under 0.1 M H₂SO₄ at 80 °C, doubling service life vs. Pt.
- Redox Flexibility: IR ladders in cyclic voltammetry show reversible Ir(III)/Ir(IV) peaks with ΔE < 50 mV, ideal for fast-charge applications .
Conclusion. Honrel’s H₂IrCl₆·6H₂O commands premium ROI where harsh conditions demand long-lived catalysts.
7. Technical Specifications Table
| Property | Value | Unit | Source |
|---|---|---|---|
| Molecular Weight | 515.04 | g·mol⁻¹ | Honrel spec |
| Density | 1.02 | g·cm⁻³ | Honrel spec |
| Melting/Decomp. Point | 65 | °C | Honrel spec |
| Solubility (Water @ 30 °C) | 456 | g·L⁻¹ | Honrel spec |
| Purity | ≥ 99 | % | Honrel spec |
| Iridium Content | 35–45 | % (wt) | Honrel spec |
| Impurity Limits (Pd, Au…) | < 0.005 | % | Honrel spec |
| Storage | 2–8 °C, inert gas | – | Honrel spec |
| UN Transport | 3260, Class 8, PG III | – | Honrel spec |
Conclusion
Hexachloroiridic acid hexahydrate, H₂IrCl₆·6H₂O, sits at the nexus of high-end catalysis, electroplating, and polyoxometalate engineering. Its robust structure, tight impurity specs, and versatile scenarios deliver measurable ROI and lifecycle gains. By choosing Honrel’s material—available in tailored packaging and guaranteed ≥ 99% purity—you tap into a trusted supply chain that fuels next-gen clean-energy, electronics, and industrial R&D.
Explore more at Honrel Catalysts and request a quote in minutes.