![]()

What is Manganese Carbonate MnCO3?
Introduction
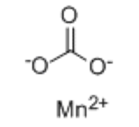
Manganese Carbonate, molecular formula: MnCO ₃, CAS: 598-62-9. It is a flexible not natural compound that takes place normally and can be generated on a large scale. Plays a crucial role throughout multiple industries. You’ll find it in plant foods, ceramics, batteries, and even concrete stains. In this review, we’ll discover its structure, homes, production routes, scenario-based uses, and safety and security factors to consider. We’ll also highlight exactly how Honrel’s high-purity manganese carbonate can reinforce your process effectiveness (Source: Wikipedia).
Our discussion unfolds in three parts: first, we’ll delve into the compound’s crystal structure and natural mineral form. Next, we’ll examine its physical and chemical attributes, followed by industrial production techniques. Then, we’ll survey the diverse scenario contexts—ranging from agriculture to advanced materials—where MnCO₃ adds value. Finally, we’ll address handling protocols and health considerations before wrapping up with key takeaways and business insights (Source: Wikipedia PubChem Honrel).
By the end, you’ll understand MnCO₃’s core traits, how it’s made, its versatile roles, and why sourcing from a trusted supplier like Honrel matters.
Table of Contents
1. Chemical Structure and Natural Mineral Form
Manganese carbonate adopts a calcite-like lattice where Mn²⁺ ions occupy octahedral sites, bonded to carbonate groups. This rhombohedral motif mirrors calcium carbonate’s structure, lending MnCO₃ its stability and dense packing.
In nature, MnCO₃ crystallizes as the mineral rhodochrosite, prized by collectors for its rose-red hues(However, for industrial needs, it’s typically manufactured). Rhodochrosite forms scalenohedral crystals with perfect cleavage, making it a distinctive trigonal system mineral.

Impurities like Fe²⁺, Ca²⁺, and Mg²⁺ often substitute into the MnCO₃ lattice, yielding color variants from pale pink to cinnamon brown. This solid-solution behavior influences both aesthetic value and ore-processing methods.
Industrial rhodochrosite feedstock typically comes from Argentina and South Africa. Processing these ores involves crushing and gravity separation to enrich MnCO₃ content. Honrel taps into such reliable sources to deliver consistent quality.

2. Key Physical and Chemical Properties
Below is a summary table of MnCO₃’s essential attributes, drawing on data from PubChem and Wikipedia.
| Property | Value | Source |
|---|---|---|
| Formula | MnCO₃ | Wikipedia, American Elements |
| Molar Mass | 114.95 g·mol⁻¹ | Wikipedia, Honrel |
| Appearance | White to faint pink solid | Wikipedia, PubChem |
| Density | 3.12 g·cm⁻³ | Wikipedia |
| Solubility | Negligible in water; soluble in acid; Soluble in dilute acids; insoluble in alcohol, ammonia | Wikipedia |
| Decomposition Temperature | ~200 °C (decomposes to MnO and CO₂) | Wikipedia |
| Crystal System | Hexagonal–rhombohedral | Wikipedia |
| Heat Capacity | 94.8 J·mol⁻¹·K⁻¹ | Wikipedia |
MnCO₃ appears as a pale powder that resists water uptake. That insolubility underpins its stability in soil and ceramic glazes Wikipedia.

When heated above 200 °C, MnCO₃ releases CO₂, leaving manganese oxide. This calcination step drives its use as a precursor in battery cathode materials and ceramic fluxes.
The compound’s refractive index (n = 1.597) and magnetic susceptibility (+11,400 × 10⁻⁶ cm³·mol⁻¹) cater to niche optical and magnetic applications. These niche metrics can affect product consistency in specialized sectors.
3. Major Production Processes
Manufacturers usually generate MnCO₃ via precipitation. They mix manganese(II) nitrate solutions with ammonia and CO₂, prompting fine carbonate floc. This process stream yields uniform particles ideal for high-end uses.
Operators control pH and temperature to tune crystal size. Lower pH favors nanoscale precipitates; higher pH yields granular solids. Adjusting these parameters helps match feedstock specs for fertilizers versus ceramics.
After precipitation, plants wash the product to remove ammonium nitrate by-product. Often, they recycle that stream back into fertilizer grade ammonium nitrate, closing the loop and cutting waste.
An alternative route uses sodium carbonate and manganese(II) chloride, which also precipitates MnCO₃. That route suits plants prioritizing low-chloride streams to reduce downstream corrosion risks.

Natural ore refining remains another path. Companies crush rhodochrosite, employ gravity or flotation separations, then recrystallize under hydrothermal conditions. This multi-stage approach yields battery-grade carbonate (Source: American Elements).
Batch reactors ensure tight residence times, while continuous setups boost throughput. Honrel leverages both models to meet diverse customer demands, from small R&D quantities to bulk supply.
4. Wide Range of Scenario Contexts
In agriculture, MnCO₃ corrects manganese deficiencies in crops. Delivering trace-nutrient supplementation improves chlorophyll synthesis, boosting yield. Farmers appreciate its slow-release profile in soil amendments.
Ceramics producers use it as a fluxing agent and colorant. When melted, MnCO₃ enhances glaze fluidity and imparts pink to brown hues. Potteries and tile makers rely on its consistent melt-behavior to avoid firing defects.
Battery manufacturers calcine MnCO₃ to MnO₂, a key component in dry-cell and lithium-ion cathodes. The controlled decomposition supports uniform mixed-metal oxide formation, critical for cycle stability (Source: ChemicalBook).
Feed formulators add MnCO₃ to livestock pellets as a bioavailable manganese source. Proper dosing prevents nutritional gaps, safeguards bone development, and supports enzyme function in animals.
Concrete stain specialists leverage its acid-reactivity. Applying dilute HCl to MnCO₃ on concrete yields custom earthy tints. This niche use case taps into decorative concrete markets looking for unique coloration (Source: Wikipedia).
Chemical plants adopt MnCO₃ as a catalyst precursor. After calcination, the resulting MnOx phases drive oxidation processes in organic synthesis. This role underscores carbonate’s upstream importance in specialty chemicals.
Honrel’s manganese carbonate addresses these varied scenarios with tailored particle sizes and purity grades. You can request custom specs along with technical support to optimize your process stream.

5. Manganese Carbonate Market Size and Growth Projections
Agriculture’s Enduring Requirement:
Impact: The farming market stays a main consumer. Manganese is a vital micronutrient, and MnCO_3 is a cost-efficient method to deliver it to soils and plants (Source: Dataintelo, Wikipedia).
Pattern: The continual demand to improve international agricultural efficiency to feed a growing population sustains sustained demand (Resource: Dataintelo). Innovations in farming like precision agriculture additionally add.
The Battery Boom:
Influence: While manganese carbonate itself isn’t straight in batteries, it’s an essential precursor for high-purity manganese sulfate (HPMSM) made use of in lithium-ion battery cathodes (NMC, LMO kinds) (Resource: Walsun New Materials).
Trend: The explosive growth of electric automobiles (EVs) and power storage space systems is a major driver. Bargains like General Motors broadening its collaboration for manganese sulfate supply highlight this fad (Source: openPR). This is pressing need for high-purity “battery-grade” manganese carbonate.
Growth in Chemical and Pharmaceutical Industries:
Influence: Made use of in manufacturing various other chemicals and as an ingredient in pharmaceuticals and dietary supplements (Source: Dataintelo).
Fad: Increasing health and wellness understanding and the growth of the pharmaceutical sector are improving need for pharmaceutical-grade MnCO_3 (Source: Dataintelo).
Manganese Carbonate Market Data
The international Manganese Carbonate Market was valued at approximately USD 270.6 million in 2022 and is predicted to reach USD 384.4 million by 2030, growing at a Substance Yearly Growth Rate (CAGR) of around 4.5% throughout the forecast duration 2024-2031 (Source: openPR, referencing DataM Intelligence).
Another report indicates the marketplace dimension was valued at USD 350 million in 2023 and is expected to reach USD 560 million by 2032, with a CAGR of 5.2% (Resource: Dataintelo). (Note: Small variations in market figures prevail between different marketing research companies because of methods and extent.).
6. Safety and Toxicity Considerations
Manganese dust poses inhalation risks; chronic exposure may lead to manganism—a neurological disorder. Facilities must enforce dust-control measures and provide respirators to workers.
Operators should store MnCO₃ in dry, ventilated areas to prevent airborne dispersion. Local exhaust ventilation and sealed transfer systems cut fugitive dust emissions, protecting both personnel and equipment.
Environmental discharge requires neutralization. Spent washwaters often contain residual Mn²⁺; treatment with hydroxide precipitates manganese hydroxide, which can be filtered out. That step aligns with effluent standards.
Regulatory bodies set workplace exposure limits, typically 0.02 mg/m³ for Mn particulate. Compliance with OSHA and EU directives keeps your site audit-ready and minimizes liability.
When handling acids to stain concrete, operators must use gloves and goggles. Acid contact with MnCO₃ releases CO₂, leading to fizzing—keep containers vented to avoid pressure buildup and potential spills.
Honrel includes material safety data sheets (MSDS) with every shipment. You’ll get clear handling instructions and waste-disposal guidelines, ensuring you maintain a safe, compliant workplace.
Conclusion
We’ve covered the core facets of manganese carbonate: its calcite-type crystal structure, pale-hued appearance, and robust chemical profile. You now see why MnCO₃ resonates in both research and manufacturing (Source: simple.wikipedia.org).
Our review of industrial routes—precipitation, ore refining, and crystallization—highlights how process controls shape particle specs. Whether in batch or continuous setups, quality hinges on tight pH and temperature management.
The scenario contexts—from crop nutrition to ceramic fluxing, battery cathodes, and decorative concrete—showcase MnCO₃’s versatility. Each use case demands precise grade and purity, which Honrel stands ready to supply.
Safety protocols for dust and acid handling protect teams and facilities. Regulatory compliance, backed by Honrel’s MSDS, keeps you on solid ground, minimizing risk and ensuring consistent product performance.
For more information or to request a quote, visit the Honrel Manganese Carbonate product page or our Catalysts page to find more related products. Partner with us to provide high-purity MnCO₃ for your next project and continuously improve your supply chain.




